LifeLabs is introducing new assay methods for therapeutic drug monitoring (TDM) tests, and for ASOT (antistreptolysin O antibodies / titer), ceruloplasmin and haptoglobin testing. For some tests this means a change in the assay platform. A major benefit from these changes is the standardization of the assay methods and reporting across all LifeLabs laboratory sites.
Overall, there will be no significant change to reported results. However we have taken this opportunity to update some of the therapeutic ranges, in accordance with current guidelines, and where appropriate, to update analyte reference intervals.
The change will take place on December 11, 2017. At that time, a message will appear on the affected lab reports indicating the details of the change for each test. Please refer to the lab report for analyte specific messages,therapeutic ranges, orreference intervals.
The following tests are affected:
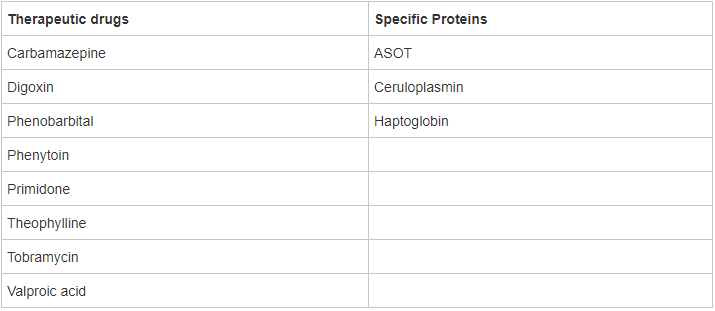
For Gentamicin, testing will be referred out to the Laboratory at the Hospital for Sick Children, Toronto.
As usual, these changes are being implemented in the context of our continuing effort to provide better quality and more reliable services to our clients. We welcome your feedback.
Please contact the LifeLabs Customer Care Centre at 1-877-849-3637 for all enquiries.
Patrick St. Louis PhD Discipline Head, Chemistry




